Critical Point Drying
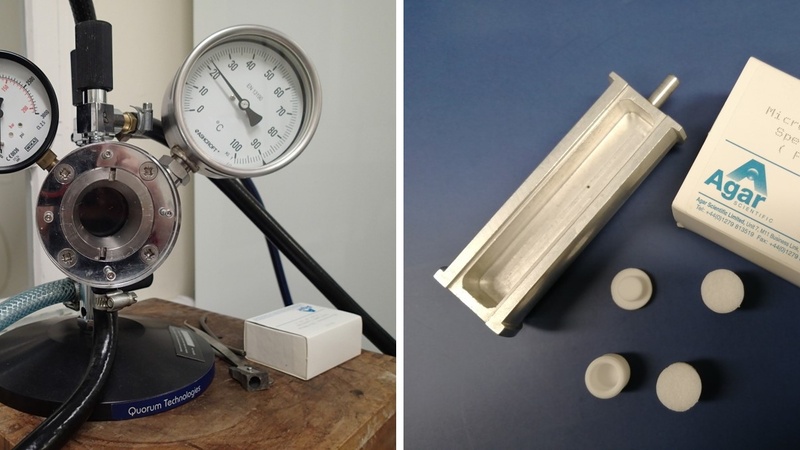
Quorum E3100 critical point drier with large specimen chamber (left). Metal specimen boats (dimensions: length ~ 75 mm, diameter ~ 12 mm) are available for drying larger samples and microporous specimen capsules are used as boat inserts for enclosing small samples (right).
The Quorum critical point dryer at CAIC is suitable for drying small as well as relatively large samples for scanning electron microscopy. Prior to CPD, biological samples tend to be fixed using chemical fixatives - such as formaldehyde and/ or glutaraldehyde for instance - while hydrogel samples are often processed unfixed. Subsequently, samples are washed in deionised water to remove fixatives and any buffer salts and are then dehydrated; we routinely use ethanol. Large samples - e.g. whole insects, larger animal/ plant tissue pieces, small embryos/ organoids/ cells attached to coverslips - can be placed directly in the specimen boat; small samples – e.g. tiny organisms, small tissue pieces or hydrogel particles – need to be enclosed in microporous specimen capsules to avoid loss of sample material during processing. Samples are inserted into the CPD chamber, flushed several times with liquid CO2 and then dried by increasing the temperature. After that, samples are ready for mounting and sputter-coating.
For some examples and more info on the process of CPD, please see below.
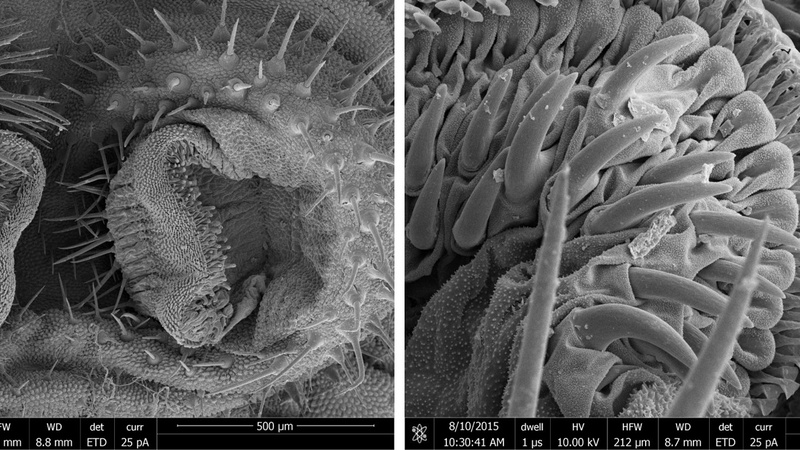
Caterpillar Bicyclus anynana, SEM of attachment structures (Simon Chen, Prof. Walter Federle Group, Dept. of Zoology, Cambridge).
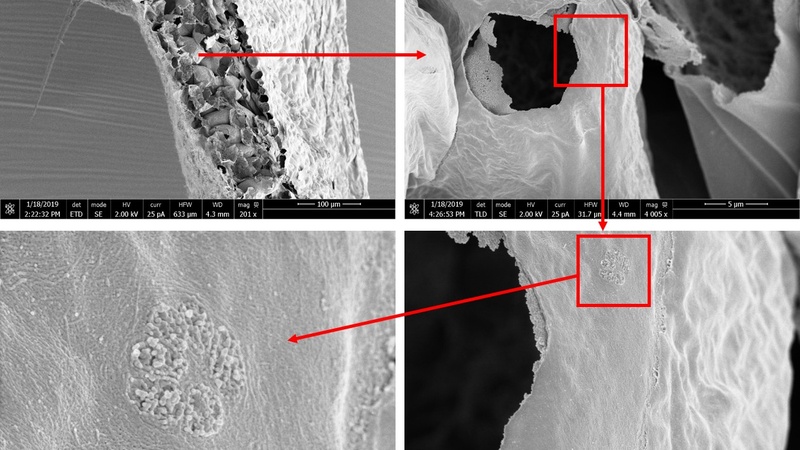
Scanning electron micrographs of a critically point dried (CPD) leaf from an Arabidopsis thaliana plant after dry fracturing. Tissue level organisation can be observed (top left panel), as well as submicron detail, such as the plasmodesmata pitfields in the cell walls (Tina Schreier, Julian Hibberd Group, Dept. of Plant Sciences, Cambridge).
Why CPD and how does it work?
In order to image water-containing specimens by electron microscopy, they have to be dried first. However, simple air-drying leads to considerable structural damage due to the high surface tension of water and the forces it exerts on structures at the liquid-air interface during the evaporation process. Critical point drying (CPD) is an established preparation technique during which a specimen is dried whilst keeping sample damage to a minimum by taking advantage of the critical phenomena.
The critical phenomena were discovered when studying the behaviour of gases at different temperatures and pressures. In simple terms: if a defined amount of gas at a given temperature is subjected to increasing pressure, its volume will become increasingly compressed. The gas will transition from its gaseous phase - via an intermediate, still compressible, vapour-liquid phase - to eventually becoming a liquid. Once liquefied, the volume will cease to change upon further pressure increases as liquids are virtually incompressible. If the same process is repeated at increasingly higher temperatures, the volume changes of the intermediate vapour-liquid phase become ever smaller, until – at a certain critical temperature and critical pressure - the gas transits directly from the gaseous to the liquid state. The values for the critical temperature and the critical pressure are characteristic for each type of gas. For most common gases, the critical temperature is either very low or very high making them unsuitable for CPD of biological samples. Carbon dioxide is usually the gas of choice as its critical temperature lies at +31.1oC (at a pressure of 1072 psi), allowing biological samples to be dried without sustaining thermal damage. Another physical characteristic of liquefied gases is crucial for CPD: as the temperature of a liquefied gas is increased, its surface tension becomes increasingly lower and its meniscus becomes flatter. As the surface tension heads towards zero, the liquid surface becomes unstable and eventually disappears. At this critical point, the gas transits directly from the liquid to the gaseous phase without exerting distorting forces at the liquid-gas interface.
So in order to perform CPD, the water contained in a sample needs to be replaced by liquefied CO2. As water and CO2 do not mix very well, a transitional step is required, which replaces the water in the sample with a solvent that does, usually ethanol or acetone. Subsequently, inside the CPD chamber, the ethanol or acetone is replaced by liquefied CO2 using several flushing steps. The system is closed off and the temperature is raised using a water bath. As both temperature and pressure increase, the critical point is reached, where the CO2 will transition from a liquid/wet state to its gaseous/dry state. With the surface tension being near zero, no distorting forces are exerted on structures sitting at the interphase during this transition; aka the sample is being dried without sustaining structural damage.
