Plunge-freezing/freeze-drying
Plunge-freezing and freeze-drying – Quorum/Emitech K775X

Manual setup for plunge-freezing in liquid ethane (left) and sample transfer via liquid nitrogen-cooled brass inserts to a LN2-cooled freeze-drier (right).
Plunge-freezing followed by freeze-drying is a quick method for preparing SEM samples of unfixed or fixed specimens. Usually, samples are briefly rinsed in cold deionised water to remove any salts from culture media or buffers and then immediately plunged into liquid ethane cooled with LN2. Once frozen, samples can be fractured under LN2 to reveal structures beneath the surface. Specimens are transferred to LN2-cooled brass inserts, which are subsequently placed in a LN2-cooled turbo freeze dryer, usually for drying overnight; then, they are ready for mounting and sputter coating prior to imaging. This method is only suitable for relatively thin samples as the freezing rate drops off with distance from the freezing liquid, leading to increased ice crystal growth and tissue damage with increasing tissue depth. It can be used for slightly larger samples if the region of interest lies at the surface.
The number of samples that can be handled in one drying run is governed by the brass inserts of the drier; 6 samples/run for larger samples; 8 samples/run for flat samples, e.g. cells on coverslips.
For more info on the use of liquefied ethane, see below.
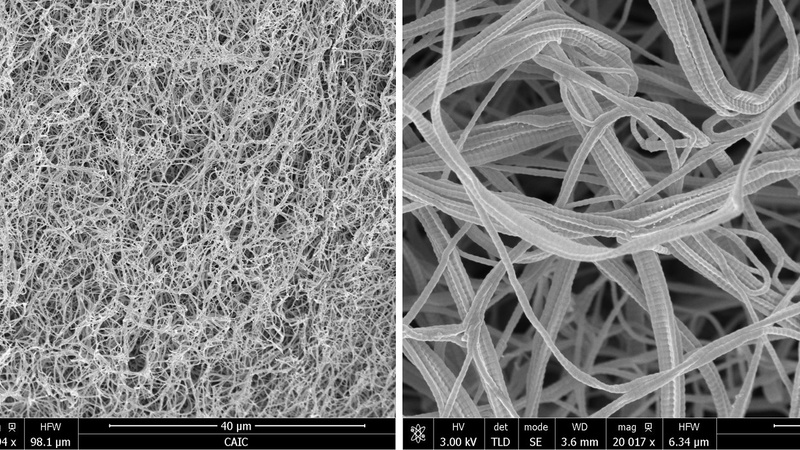
Artificial scaffold engineered from reformed collagen mimicking the extracellular matrix intended as 3D growth matrix for cancer cells (Rakesh Rajan, Prof. Melinda Duer Group, Yusuf Hamied Dept. of Chemistry, Cambridge).
Why use liquid ethane for freezing?
Liquid nitrogen is extremely cold and yet unsuitable for freezing tissues for microscopy because of its low heat capacity. Low heat capacity means only a small amount of energy needs to be transferred to LN2 to drastically raise its temperature. So, upon coming into contact with a specimen much warmer than itself, LN2 – with a boiling point of about -196oC - starts to boil immediately. An insulating layer of nitrogen gas forms between the liquid and the specimen, preventing it from freezing quickly. The slow freezing rate allows the water contained in the specimen to form ice crystals, which results in extensive tissue damage.
In contrast, liquid ethane has a much higher heat capacity. For plunge-freezing with ethane, LN2 is used to liquefy ethane gas and cool it down to almost LN2 temperatures. With a melting point of about -183oC, ethane will eventually solidify if left cooling for too long. Upon plunging the specimen, the ethane temperature does not raise as dramatically as LN2 due to its higher heat capacity. Furthermore, with a boiling point of -88.5oC, ethane will start boiling much less rapidly and no insulating gas layer is formed between the liquid and the specimen, resulting in a very fast freezing rate. The water in the specimen does not form ice crystals, but undergoes vitrification, forming an amorphous, glass-like phase, a process which leaves the tissues intact. It is important to keep temperatures below about -90oC all through the procedure of freezing, transfer and drying to avoid ice re-crystallization in the sample.
The extremely fast freezing rate of liquid ethane also explains why it should always be handled with extreme care! Even small splashes of ethane will lead to frostbite and burns, whereas small splashes of LN2 will simply evaporate off.
