SE-imaging - coated samples
Secondary electron imaging - coated samples
This is the most commonly used imaging modality in scanning electron microscopy used to visualize the surface of a specimen. Following preparation and mounting, the sample is sputter-coated with metals, such as gold, platinum, iridium or chromium. The metal coating has several effects. It improves sample conductivity minimising charging effects during imaging, and it increases the yield of secondary electrons improving the signal-to-noise ratio (elements with high atomic number produce more secondary electrons than elements with low atomic number). In addition, it creates a layer of homogeneous elemental composition on the surface of the specimen, “overriding” differences in elemental composition inherent in the specimen. Thus, the yield of surface secondary electrons becomes largely dependent on the surface topography of the specimen resulting in three-dimensional images, potentially with a large depth-of-field.
SEM imaging can cover an amazing range of magnifications as the images below illustrate:
Drosophila intestinal tract (Golnar Kohlagar, Department of Physiology, Development and Neuroscience, Cambridge). The top overview image is a stitched map created using FEI MAPS software. Successive images are increasingly zooming in on the structures denoted by the coloured squares.
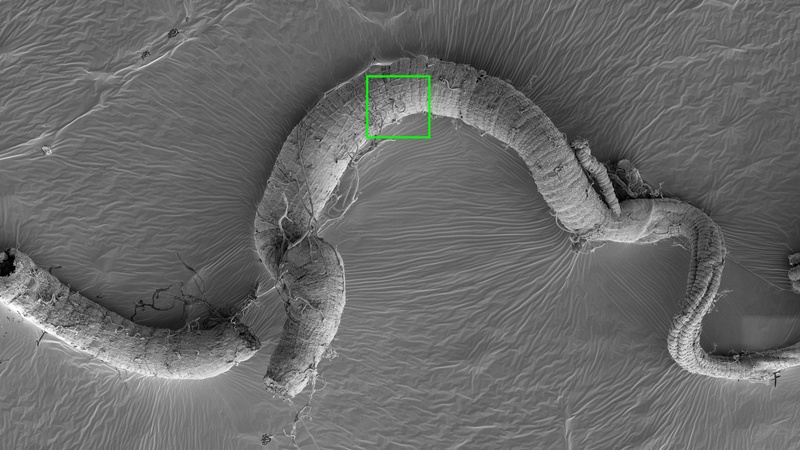

Green square: longitudinal and circular muscle layers on the gut surface
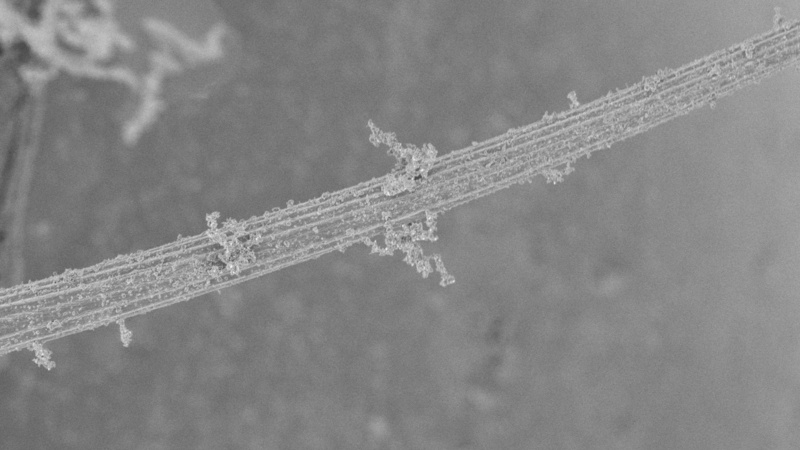
Blue square: Mechanosensory bristle
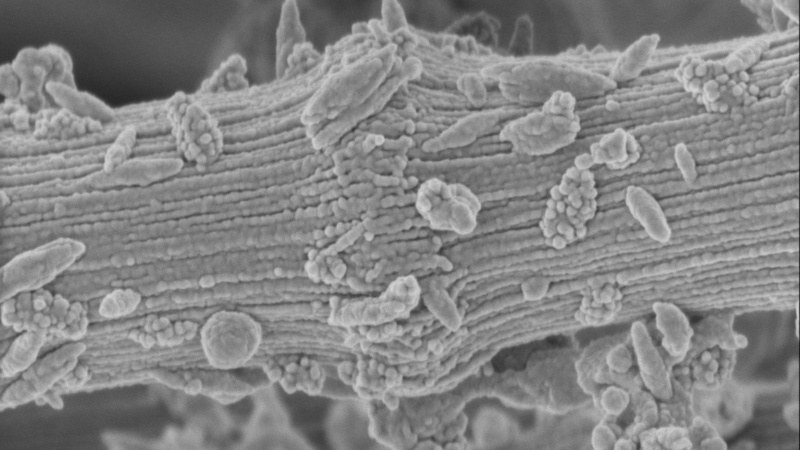
Red square: ordered fibrillar structures near the crop
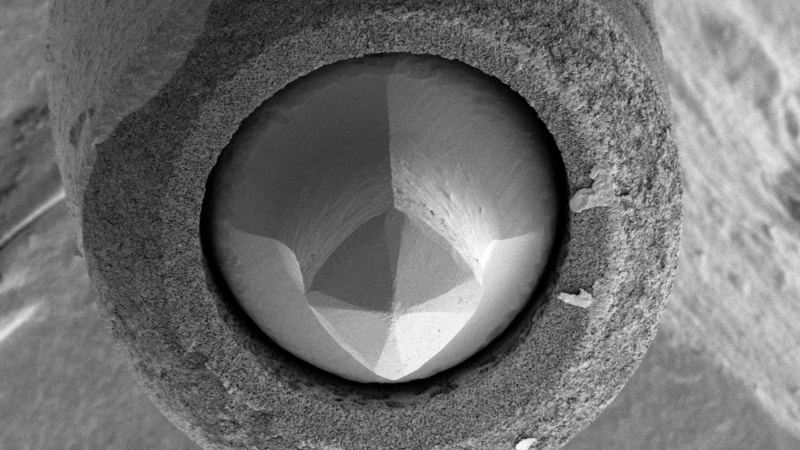
Lanthanum hexaboride (LaB6) cathode (Kimball Physics Inc.) after about 2 ½ years use as the electron source in the CAIC Tecnai TEM. The LaB6 crystal at the centre is held in place by a carbon ferrule (round, surrounding structure) and shows shortening and pitting due to extended use (Karin Müller, CAIC).
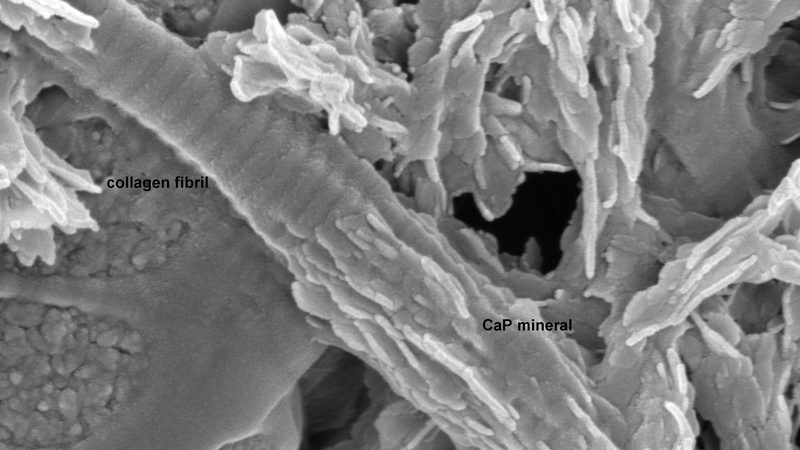
Bovine tendon collagen fibril during mineralization with a calcium phosphate mineral; collagen banding is clearly visible in the unmineralized part of the fiber (Karin Müller, Prof. Melinda Duer Group, Yusuf Hamied Department of Chemistry, Cambridge).
