Negative Staining
Negative staining
One of the quickest and easiest methods of specimen preparation for TEM is to use electron-dense stains (like uranyl acetate, ammonium molybdate or phosphotungstic acid amongst others) to contrast specimens adsorbed onto TEM grids. Negative staining is particularly suited for imaging larger proteins, protein aggregates, filaments, fibres, membrane vesicles, bacteria or viruses. It can be combined with immunostaining – using a primary antibody and a gold-labelled secondary antibody – to reveal the morphology of a biological specimen as well as testing for the presence of a particular antigen on the specimen surface.
MSPA55 bacteriophages isolated from P. aeruginosa with long tails, which can be seen here in the contracted and non-contracted forms (Maria Stroyakovski, Prof. George PC Salmond group, Biochemistry, Cambridge).
Bacterium with flagellum and gas vesicles (Rita Monson, Prof. George PC Salmond group, Biochemistry, Cambridge).
Bovine tendon collagen stained with uranyl acetate revealing the regular pattern of overlap and gap zones with finer sub-banding (Karin Müller, Prof. Melinda Duer group, Chemistry, Cambridge).
Poly(ADP) ribose in the absence and presence of calcium ions (Karin Müller, Prof. Melinda Duer group, Chemistry, Cambridge).
Exosomes isolated from cell culture supernatant: immuno-staining using the exosome marker anti-CD63 and a secondary antibody tagged with 15 nm gold particles (arrows), followed by uranyl acetate staining (Karin Müller, Prof. Catherine Shanahan, King’s College, London).
Parapox virus found in skin scrapings of infected sheep (Emilie Cloup, then Queen's Veterinary School Hospital, Cambridge).
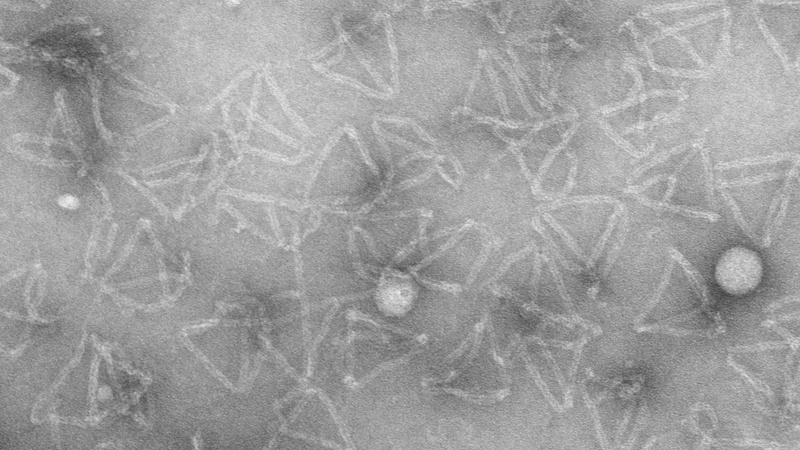
Negative staining of DNA origami tetrahedron structures with uranyl acetate (Anna Scheeder and Ioanna Mela, Prof. Clemens Kaminski Group, Dept. of Chemical Engineering and Biotechnology, Cambridge University).
